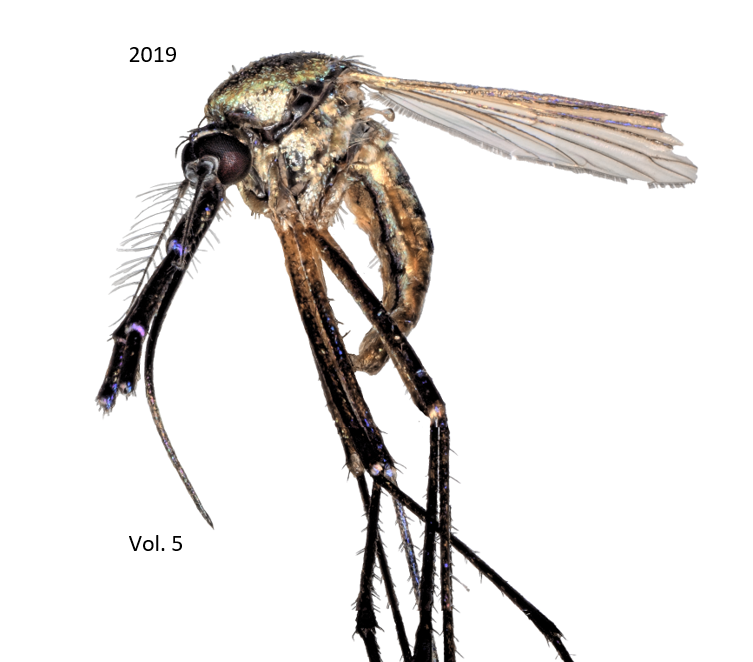
Impact of salinity, pollutants and organic debris in still water on the life cycle of larval Culex sp and the oviposition site preference of mosquitoes in Bryan-College Station, Texas
Abstract
After major natural disasters, the number of vector-borne diseases increases significantly. In light of recent events in Houston, Texas and surrounding coastal cities due to Hurricane Harvey, a study was created to examine the effects of organic and inorganic pollutants on the breeding site preference and life cycle of mosquitoes. For each part of this experiment, containers of water were prepared with various organic and inorganic pollutants. To study the breeding site preference, these containers were placed outdoors in three residential properties in the Bryan-College Station, Texas community. After a period of 27 days, no data was observed as the cold, frigid weather in the city was not conducive to breeding. In a second study involving the life cycle of mosquitoes, containers containing these same inorganic and organic pollutants were placed indoors. Larval Culex sp mosquitoes were observed for a period of eight days to see how each environment affected their progression. The containers with tap water, distilled water, and organic material saw the most growth of mosquitos, while the containers filled with oil and sodium chloride killed off all of the larval mosquitoes. It was concluded that neither seawater nor an aqueous environment in which there is substantial pollution, specifically oil, are conducive to mosquito growth. However, an overall generalization cannot be made on the effect of pollutants on the life cycle of all mosquitos. Further research will be needed, as the sample size was relatively small and only included one genera of mosquito.
References
Awolola, T.S., A.O. Oduola, J.B. Obansa, N.J. Chukwurar, and J.P. Unyimadu. 2007. Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. J. Vector. Borne. Dis. 44: 241-4.
Caillouet, K.A., S.R. Michaels, X. Xiong, I. Foppa, and D.M. Wesson. 2008. Increase in West Nile neuroinvasive disease after Hurricane Katrina. Emerg. Infect. Dis. 14: 804-807.
DeLisi, N., J. Ottea, and K. Healy. 2017. Susceptibility of Culex quinquefasciatus (Diptera: Culicidae) in southern Louisiana to larval insecticides. J. Econ. Entomol. 20: 1-6.
Estallo, E.L., F.F. Luduena-Almeida, M.V. Introini, M. Zaidenberg, and W.R. Almiron. 2015. Weather variability associated with Aedes (stegomyia) aegypti (Dengue vector) oviposition dynamics in northwestern Argentina. Plos. One. 10: e0127820.
Esworthy, R., L. Schierow, C. Copeland, L. Luther, and J.L. Ramseur. 2006. Cleanup after Hurricane Katrina: environmental considerations. Library of Congress and Congressional Research Service, DC, USA.
Fillinger, U., G. Sonye, G.F. Killeen, B.G. Knols, and N. Becker. 2004. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop. Med. Int. Health. 9: 1274-1289.
Gagnon A.S., K.E. Smoyer-Tomic, and A.B. Bush. 2002. The El Nino southern oscillation and malaria epidemics in South America. Int. J. Biometeorol. 46: 81-89.
Imam, H., Zarnigar, G. Sofi, and A. Seikh. 2014.The basic rules and methods of mosquito rearing (Aedes aegypti). Trop. Parasitol. 4: 53-55.
Jonusaite, S., A. Donini, and S.P. Kelly. 2017. Salinity alters snakeskin and mesh transcript abundance and permeability in midgut and malpighian tubules of larval mosquito, Aedes aegypti. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 205: 58-67.
Lee, F.C. 1973. Effect of various sodium chloride concentrations on the development of the mosquito Culiseta incidens (Thomson) (Diptera: Culicidae). Mosq. News. 33: 78-83.
Mains, J.W., D.R. Mercer, and S.L. Dobson. 2008. Digital image analysis to estimate numbers of Aedes eggs oviposited in containers. J. Am. Mosq. Control. Assoc. 24: 496-501.
Panigrahi S.K., T.K. Barik, S. Mohanty, and N.K. Tripathy. 2014 Laboratory evaluation of oviposition behavior of field collected Aedes mosquitoes. J. Insects. 2014: 1-8.
Riccuiti, E. 2016. GAT mosquito traps can be effective even without pesticides. Entomology Today. Entomological Society of America, retrieved September 28, 2017, from https://entomologytoday.org
Roberts, D.M. and R.J. Irving-Bell. 1997. Salinity and microhabitat preferences in mosquito larvae from southern Oman. J. Arid. Environ. 37: 497-504.
Saeed, U. and Z.Z. Piracha. 2016. Viral outbreaks and communicable health hazards due to devastating floods in Pakistan. World. J. Virol. 5: 82-84.
Shaman, J., M. Stieglitz, C. Stark, S. Le Blancq, M. Cane. 2002. Using a dynamic hydrology model to predict mosquito abundances in flood and swamp water. Emerg. Infect. Dis. 8: 8-13.
Steyer, G.D., B.C. Perez, S. Piazza, G. Suir. 2007. Potential consequences of saltwater intrusion associated with hurricanes katrina and rita, pp. 137-146. In G.S. Farris, et al. (eds.), Science and the storms-the USGS response to the hurricanes of 2005. U.S. Geological Survey, LA.
US Climate Data. 2017. Climate College Station - Texas. Retrieved September 28, 2017, from https://www.usclimatedata.com.
Wang, Y., W. Pons, J. Fang, and H. Zhu. 2017. The impact of weather and storm water management ponds on the transmission of West Nile virus. R. Soc. Open. Sci. 4: 170017.
Wigglesworth, V.B. 1932. The adaptation of mosquito larvae to salt water. J. Exp. Biol. 10: 27-36.
Downloads
Published
Issue
Section
License
Instars supports the need for authors to share, disseminate and maximise the impact of their research. We take our responsibility as stewards of the online record seriously, and work to ensure our policies and procedures help to protect the integrity of scholarly works.
License to the Journal. The Author hereby licenses to the Journal the irrevocable, nonexclusive, and royalty-free rights as follows:
a. The Journal may publish the Article in any format, including electronic and print media. Specifically, this license include the right to reproduce, publicly distribute and display, and transmit the Article or portions thereof in any manner, through any medium now in existence or developed in the future, including but not limited to print, electronic, and digital media, computerized retrieval systems, and other formats
b. The Journal may prepare translations and abstracts and other similar adaptations of the Article in furtherance of its publication of the Article.
c. The Journal may use the Author's name, likeness, and institutional affiliation in connection with any use of the Article and in promoting the Article or the Journal.
d. The Journal may exercise these rights directly or by means of third parties. The Journal may authorize third-party publishers, aggregators, and printers to publish the Article or to include the Article in databases or other services. [Examples of such third parties include Westlaw, Lexis, and EBSCO.]
e. The Journal may without further permission from the Author transfer, assign, or sublicense the rights that the Journal has pursuant to the Agreement.
f. In order to foster wider access to the Article, especially for the benefit of the nonprofit community, the Author hereby grants to the Journal the authority to publish the Article with a Creative Commons "Attribution, Non-Commercial, No Derivatives" license. [The Author should consult the Creative Commons website (www.creativecommons.org) for further information]
g. This liscense of rights to the Journal shall take effect immediately. In the event that the Journal does not publish the Article, this license to the Journal shall temrinate upon written notification by the Journal to the Author, or upon termination of all publication by the Journal. To the extent that moral rights may apply to the Article, this agreement does not affect the moral rights of the Author in or to the Article.
Rights of the Author. Without suggesting any limit on other rights that the Author may h ave with respect to the Article, the Author retains the following rights. To the extent that the Journal holds similar rights with respect to the Article consistent with this Agreement, the Author shall hold these rights on a nonexclusive basis. To the extent that the Article includes edits and other contributions by the staff of the Journal, the rights of the Author in this Paragraph include the right to use such edits and contributions.
a. The Author may publish the Article in another scholarly journal, in a book, or by other means. The Author may exercise this right of publication only after the date of first publication of the ARticle in the Journal in any format.
b. The Author shall, without limitation, have the right to use the ARticle in any form or format in connection with the Author's teaching, conference presentations, lectures, other scholarly works, and for all of Author's academic and professional acitvities.
c. The Author shall at any time have the fright to make, or to authorize others to make, a preprint or a final published version of the ARticle available in digital form over the Internet, including, but not limited to, a website under the control fo the Author or the Author's employer or through digital repositories including, but not limited to, those maintained by scholarly societies, funding agencies, or the Author's employer. This right shall include, without limitation, the right of the Author to permit public access to the Article as part of a repository or through a service or domain maintained by teh Author's employing the institution or a service as required by law or by agreement with a funding agency. The Journal may in its discretion deposit the ARticle with any digital repository consisten with deposits permitted by the Author under ths paragraph. [Examples of such repositories include SSRN, arXiv.org, PubMed Central, and Academic Commons at Columbia University.]
d. Any of the foregoing permitted uses of the Article, or of a work based substantially on the Article, shall include an appropriate citation to the Article, stating that it has been or is to be published in the Journal, with name and date of the Journal publication and the Internet address for the website of the Journal.
Editing of the Article. This Agreement is subject to the understanding that the ordinary editing processes of the Journal will be diligently pursued and that the Article will not be published by the Journal unless, in tis final form, it is acceptable to the Author and the Journal.